Teltron tube
From Wikipedia, the free encyclopedia
Teltron electron beam tube
A
teltron tube (named for Teltron Inc., which is now owned by 3B Scientific Ltd.) is a type of
cathode ray tube used to demonstrate the properties of
electrons. It usually contains two
electron guns,
which can project two thin electron beams at right angles. The beams
can be bent by applying voltages to various electrodes in the tube. The
electron beams are visible as fine bluish lines. This is accomplished by
filling the tube with low pressure
helium (He) or
Hydrogen (H
2) gas. A few of the electrons in the beam collide with the helium atoms, causing them to
fluoresce and emit light.
They are usually used to teach electromagnetic effects because they show how an electron beam is affected by
electric fields and by
magnetic fields like the
Lorentz force.
Motions in fields
Charged particles in a uniform electric field follow a
parabolic trajectory, since the
electric field term (of the
Lorentz force
which acts on the particle) is the product of the particle's charge and
the magnitude of the electric field, (oriented in the direction of the
electric field). In a uniform magnetic field however, charged particles
follow a circular trajectory due to the
cross product in the
magnetic field
term of the Lorentz force. (That is, the force from the magnetic field
acts on the particle in a direction perpendicular to the particle's
direction of motion. See:
Lorentz force for more details.)
Apparatus
The 'teltron' apparatus consists of a Teltron type electron deflection tube, a Teltron stand,
EHT power supply (
0 - 5000 V DC, variable).
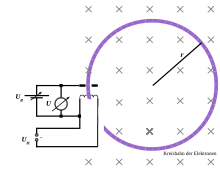
Experimental setup
Experimental sketch fine beam
In an evacuated glass bulb some
hydrogen gas (H
2) is filled, so that the tube has a hydrogen atmosphere at low pressure of about
1 Pa
is formed. The pressure is such that the electrons are decelerated by
collisions as little as possible (change in kinetic energy), the number
of collisions are few but sufficient to emit visible light. Inside the
bulb there is an
electron gun. This consists of a heating spiral, a cathode and an anode hole. From the
cathode (
-) electrons are emitted and accelerated by the electric field towards the positively charged anode (
+). Through a hole in the anode, the electrons leave the beam forming system and the
Wehnelt cylinder bundles.
Results
Another experimental setup showing the electron beam orbit
When the heater is energized, the heating coil will cause electrons to emerge from it due to
thermionic emission. In the
electric field
between anode and cathode, the electric field acts on the electrons,
which accelerate to a high velocity, such that the electrons leave
through a small opening in the anode as an electron beam. Only when the
coil current is turned on will a force act on the beam and change its
direction. Otherwise it will retain its velocity. If, however, the
coil current is switched on, the
Lorentz force will direct the electrons into a circular orbit.
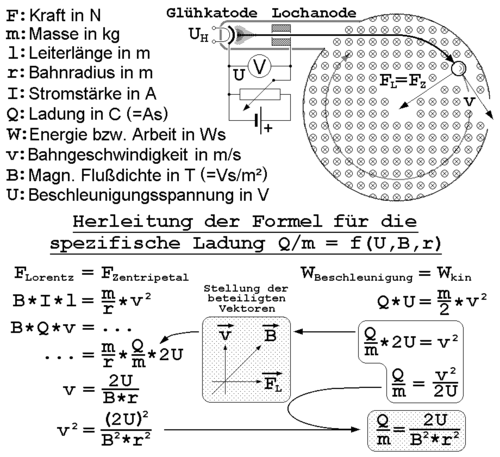
Determination of the specific electron charge
Derivation of the formula to determine the specific charge of the electron with the help of the filament beam-test
The higher the coil current, the stronger magnetic field and thus
smaller radius of the circular path of the electrons. The strength of
the magnetic field and the
Lorentz force
are proportional to each other, such that when the Lorentz force
increases. A larger Lorentz force will deflect the electrons more
strongly, so the orbit will be smaller. The Lorentz force

is always perpendicular to the instantaneous direction of movement and allows a centripetal

circular motion. The magnitude of the velocity and hence the kinetic energy can not change:

From this we get the amount of specific electron charge

The determination of the velocity is performed using the
energy conservation law

This is finally followed by

The specific electron charge has the value

Since the
charge of an electron is available from the
Millikan experiment, the study of electrons in an magnetic field is the determination of its
mass in accordance with:

Similar concepts for the weighing of charged particles can be found in the
mass spectrometer.
External links
 is always perpendicular to the instantaneous direction of movement and allows a centripetal
is always perpendicular to the instantaneous direction of movement and allows a centripetal  circular motion. The magnitude of the velocity and hence the kinetic energy can not change:
circular motion. The magnitude of the velocity and hence the kinetic energy can not change:








No comments:
Post a Comment