Magnetic moment
From Wikipedia, the free encyclopedia
| Electromagnetism |
|---|
 |
The magnetic moment may be considered to be a vector having a magnitude and direction. The direction of the magnetic moment points from the south to north pole of the magnet. The magnetic field produced by the magnet is proportional to its magnetic moment. More precisely, the term magnetic moment normally refers to a system's magnetic dipole moment, which produces the first term in the multipole expansion of a general magnetic field. The dipole component of an object's magnetic field is symmetric about the direction of its magnetic dipole moment, and decreases as the inverse cube of the distance from the object.
Contents
Definition
The magnetic moment is defined as a vector relating the aligning torque on the object from an externally applied magnetic field to the field vector itself. The relationship is given by[1] is the torque acting on the dipole and
is the torque acting on the dipole and  is the external magnetic field, and
is the external magnetic field, and  is the magnetic moment.
is the magnetic moment.This definition is based on how one would measure the magnetic moment, in principle, of an unknown sample.
Units
The unit for magnetic moment is not a base unit in the International System of Units (SI). As the torque is measured in newton-meters (N·m) and the magnetic field in teslas (T), the magnetic moment is measured in newton-meters per tesla. This has equivalents in other base units:In the CGS system, there are several different sets of electromagnetism units, of which the main ones are ESU, Gaussian, and EMU. Among these, there are two alternative (non-equivalent) units of magnetic dipole moment:
 (ESU)
(ESU) (Gaussian and EMU),
(Gaussian and EMU),
All formulae in this article are correct in SI units; they may need to be changed for use in other unit systems. For example, in SI units, a loop of current with current I and area A has magnetic moment IA (see below), but in Gaussian units the magnetic moment is IA/c.
Two representations of the cause of the magnetic moment
The preferred classical explanation of a magnetic moment has changed over time. Before the 1930s, textbooks explained the moment using hypothetical magnetic point charges. Since then, most have defined it in terms of Ampèrian currents.[2] In magnetic materials, the cause of the magnetic moment are the spin and orbital angular momentum states of the electrons, and whether atoms in one region are aligned with atoms in another.Magnetic pole representation
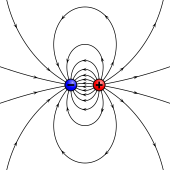
An electrostatic analog for a magnetic moment: two opposing charges separated by a finite distance.
Integral representation
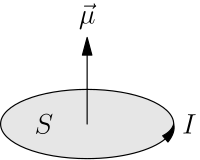
Moment μ of a planar current having magnitude I and enclosing an area S
The integral magnetic moment of a charge distribution is therefore:
 ,
,
On the other hand for a point particle the angular momentum is defined as:
 ,
,
Magnetic moment of a solenoid
Image of a solenoid
Magnetic moment and angular momentum
The magnetic moment has a close connection with angular momentum called the gyromagnetic effect. This effect is expressed on a macroscopic scale in the Einstein-de Haas effect, or "rotation by magnetization," and its inverse, the Barnett effect, or "magnetization by rotation."[1] In particular, when a magnetic moment is subject to a torque in a magnetic field that tends to align it with the applied magnetic field, the moment precesses (rotates about the axis of the applied field). This is a consequence of the concomitance of magnetic moment and angular momentum, that in case of charged massive particles corresponds to the concomitance of charge and mass in a particle.Viewing a magnetic dipole as a rotating charged particle brings out the close connection between magnetic moment and angular momentum. Both the magnetic moment and the angular momentum increase with the rate of rotation. The ratio of the two is called the gyromagnetic ratio and is simply the half of the charge-to-mass ratio.[4] [5]
For a spinning charged solid with a uniform charge density to mass density ratio, the gyromagnetic ratio is equal to half the charge-to-mass ratio. This implies that a more massive assembly of charges spinning with the same angular momentum will have a proportionately weaker magnetic moment, compared to its lighter counterpart. Even though atomic particles cannot be accurately described as spinning charge distributions of uniform charge-to-mass ratio, this general trend can be observed in the atomic world, where the intrinsic angular momentum (spin) of each type of particle is a constant: a small half-integer times the reduced Planck constant ħ. This is the basis for defining the magnetic moment units of Bohr magneton (assuming charge-to-mass ratio of the electron) and nuclear magneton (assuming charge-to-mass ratio of the proton).
Effects of an external magnetic field on a magnetic moment
Force on a moment
See also: force between magnets
A magnetic moment in an externally produced magnetic field has a potential energy U:An electron, nucleus, or atom placed in a uniform magnetic field will precess with a frequency known as the Larmor frequency. See Resonance.
Magnetic dipoles
Main article: Magnetic dipole
See also: Dipole
A magnetic dipole is the limit of either a current loop or a pair of
poles as the dimensions of the source are reduced to zero while keeping
the moment constant. As long as these limits only apply to fields far
from the sources, they are equivalent. However, the two models give
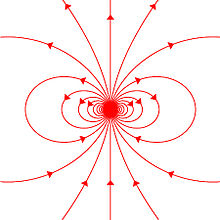
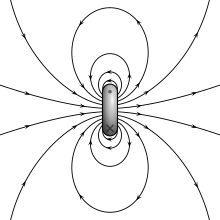
different predictions for the internal field (see below).External magnetic field produced by a magnetic dipole moment
Magnetic field lines around a "magnetostatic dipole". The magnetic
dipole itself is located in the center of the figure, seen from the
side, and pointing upward.
The vector potential of magnetic field produced by magnetic moment m is
Internal magnetic field of a dipole
The magnetic field of a current loop
If a magnetic dipole is formed by making a current loop smaller and smaller, but keeping the product of current and area constant, the limiting field is
If a magnetic dipole is formed by taking a "north pole" and a "south pole", bringing them closer and closer together but keeping the product of magnetic pole-charge and distance constant, the limiting field is[2]
 , where
, whereForces between two magnetic dipoles
See also: Magnetic dipole-dipole interaction
As discussed earlier, the force exerted by a dipole loop with moment m1 on another with moment m2 isThe torque of magnet 1 on magnet 2 is
Examples of magnetic moments
Two kinds of magnetic sources
Fundamentally, contributions to any system's magnetic moment may come from sources of two kinds: (1) motion of electric charges, such as electric currents, and (2) the intrinsic magnetism of elementary particles, such as the electron.Contributions due to the sources of the first kind can be calculated from knowing the distribution of all the electric currents (or, alternatively, of all the electric charges and their velocities) inside the system, by using the formulas below. On the other hand, the magnitude of each elementary particle's intrinsic magnetic moment is a fixed number, often measured experimentally to a great precision. For example, any electron's magnetic moment is measured to be −9.284764×10−24 J/T.[10] The direction of the magnetic moment of any elementary particle is entirely determined by the direction of its spin, with the negative value indicating that any electron's magnetic moment is antiparallel to its spin.
The net magnetic moment of any system is a vector sum of contributions from one or both types of sources. For example, the magnetic moment of an atom of hydrogen-1 (the lightest hydrogen isotope, consisting of a proton and an electron) is a vector sum of the following contributions:
- the intrinsic moment of the electron,
- the orbital motion of the electron around the proton,
- the intrinsic moment of the proton.
Magnetic moment of an atom
For an atom, individual electron spins are added to get a total spin, and individual orbital angular momenta are added to get a total orbital angular momentum. These two then are added using angular momentum coupling to get a total angular momentum. The magnitude of the atomic dipole moment is then[11] .
.
Due to the angular momentum, the dynamics of a magnetic dipole in a magnetic field differs from that of an electric dipole in an electric field. The field does exert a torque on the magnetic dipole tending to align it with the field. However, torque is proportional to rate of change of angular momentum, so precession occurs: the direction of spin changes. This behavior is described by the Landau-Lifshitz-Gilbert equation:[14][15]
 is gyromagnetic ratio, m is magnetic moment, λ is damping coefficient and Heff
is effective magnetic field (the external field plus any self-field).
The first term describes precession of the moment about the effective
field, while the second is a damping term related to dissipation of
energy caused by interaction with the surroundings.
is gyromagnetic ratio, m is magnetic moment, λ is damping coefficient and Heff
is effective magnetic field (the external field plus any self-field).
The first term describes precession of the moment about the effective
field, while the second is a damping term related to dissipation of
energy caused by interaction with the surroundings.Magnetic moment of an electron
See also: Anomalous magnetic dipole moment
Electrons and many elementary particles also have intrinsic magnetic moments, an explanation of which requires a quantum mechanical treatment and relates to the intrinsic angular momentum of the particles as discussed in the article Electron magnetic moment. It is these intrinsic magnetic moments that give rise to the macroscopic effects of magnetism, and other phenomena, such as electron paramagnetic resonance.The magnetic moment of the electron is
Again it is important to notice that m is a negative constant multiplied by the spin, so the magnetic moment of the electron is antiparallel to the spin. This can be understood with the following classical picture: if we imagine that the spin angular momentum is created by the electron mass spinning around some axis, the electric current that this rotation creates circulates in the opposite direction, because of the negative charge of the electron; such current loops produce a magnetic moment which is antiparallel to the spin. Hence, for a positron (the anti-particle of the electron) the magnetic moment is parallel to its spin.
Magnetic moment of a nucleus
See also: Nuclear magnetic moment
The nuclear system is a complex physical system consisting of nucleons, i.e., protons and neutrons.
The quantum mechanical properties of the nucleons include the spin
among others. Since the electromagnetic moments of the nucleus depend on
the spin of the individual nucleons, one can look at these properties
with measurements of nuclear moments, and more specifically the nuclear
magnetic dipole moment.Most common nuclei exist in their ground state, although nuclei of some isotopes have long-lived excited states. Each energy state of a nucleus of a given isotope is characterized by a well-defined magnetic dipole moment, the magnitude of which is a fixed number, often measured experimentally to a great precision. This number is very sensitive to the individual contributions from nucleons, and a measurement or prediction of its value can reveal important information about the content of the nuclear wave function. There are several theoretical models that predict the value of the magnetic dipole moment and a number of experimental techniques aiming to carry out measurements in nuclei along the nuclear chart.
Magnetic moment of a molecule
Any molecule has a well-defined magnitude of magnetic moment, which may depend on the molecule's energy state. Typically, the overall magnetic moment of a molecule is a combination of the following contributions, in the order of their typical strength:- magnetic moments due to its unpaired electron spins (paramagnetic contribution), if any
- orbital motion of its electrons, which in the ground state is often proportional to the external magnetic field (diamagnetic contribution)
- the combined magnetic moment of its nuclear spins, which depends on the nuclear spin configuration.
Examples of molecular magnetism
- Oxygen molecule, O2, exhibits strong paramagnetism, due to unpaired spins of its outermost two electrons.
- Carbon dioxide molecule, CO2, mostly exhibits diamagnetism, a much weaker magnetic moment of the electron orbitals that is proportional to the external magnetic field. The nuclear magnetism of a magnetic isotope such as 13C or 17O will contribute to the molecule's magnetic moment.
- Hydrogen molecule, H2, in a weak (or zero) magnetic field exhibits nuclear magnetism, and can be in a para- or an ortho- nuclear spin configuration.
- Many transition metal complexes are magnetic. The spin-only formula is a good first approximation for high-spin complexes of first-row transition metals.[16]
-
Number of
unpaired
electronsSpin-only
moment /μB1 1.73 2 2.83 3 3.87 4 4.90 5 5.92
Elementary particles
In atomic and nuclear physics, the Greek symbol μ represents the magnitude of the magnetic moment, often measured in Bohr magnetons or nuclear magnetons, associated with the intrinsic spin of the particle and/or with the orbital motion of the particle in a system. Values of the intrinsic magnetic moments of some particles are given in the table below:| Particle | Magnetic dipole moment in SI units (J⋅T−1) |
Spin quantum number (dimensionless) |
|---|---|---|
| electron | −9284.764 × 10−27 | 1/2 |
| proton | 14.106067 × 10−27 | 1/2 |
| neutron | −9.66236 × 10−27 | 1/2 |
| muon | −44.904478 × 10−27 | 1/2 |
| deuteron | 4.3307346 × 10−27 | 1 |
| triton | 15.046094 × 10−27 | 1/2 |
| helion | −10.746174 × 10−27 | 1/2 |
| alpha particle | 0 | 0 |




















![\mathbf{B}(\mathbf{x})=\frac{\mu_0}{4\pi}\left[\frac{3\mathbf{n}(\mathbf{n}\cdot \mathbf{m})-\mathbf{m}}{|\mathbf{x}|^3} + \frac{8\pi}{3}\mathbf{m}\delta(\mathbf{x})\right].](https://upload.wikimedia.org/math/4/1/9/4190c5212303ce991c1c891e0543069a.png)
![\mathbf{H}(\mathbf{x}) =\frac{1}{4\pi}\left[\frac{3\mathbf{n}(\mathbf{n}\cdot \mathbf{m})-\mathbf{m}}{|\mathbf{x}|^3} - \frac{4\pi}{3}\mathbf{m}\delta(\mathbf{x})\right].](https://upload.wikimedia.org/math/2/8/f/28fb7bf3050a637288e97abce7fc57fa.png)









No comments:
Post a Comment